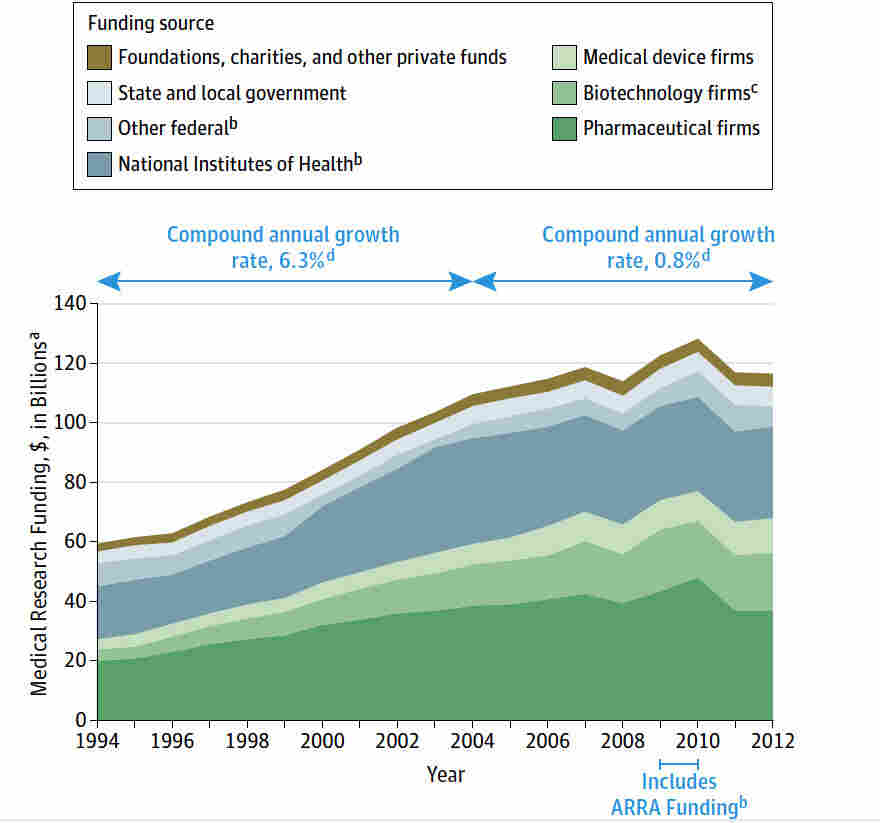
Medical research funding is crucial to advancing healthcare innovations and ensuring patient safety in research. The recent halt in funding has raised alarm about the potential impact of funding cuts on the rights of participants involved in critical studies. As federal research grants are frozen or canceled, the already intricate balance of IRB oversight is jeopardized, risking the integrity of patient safety protocols. Institutions like Harvard, which are at the forefront of national medical research, depend heavily on these funds to maintain ethical standards and rigorous oversight mechanisms. Without adequate financial support, the progress made in medical research could stall, negatively affecting the lives of countless patients who rely on breakthroughs in treatment and care.
Funding for medical research refers to the financial support provided to institutions and organizations for conducting health-related studies. This financial backing from federal sources is essential for fostering innovative research projects and safeguarding the well-being of individuals participating in medical investigations. The adverse effects of reduced funding can lead to a compromise in the oversight responsibilities of Institutional Review Boards (IRBs), which are vital for ensuring ethical standards in research. Alternative terms such as biomedical funding and clinical research financing can help broaden the discussion about the growing concerns regarding funding cuts and their ramifications on patient safety and participant rights in studies. The collaboration between research entities like Harvard and federal grant programs plays a pivotal role in shaping healthcare advancements.
The Consequences of Funding Cuts on Patient Safety in Research
The ongoing funding cuts to medical research create a ripple effect that severely compromises patient safety and the integrity of research processes. With each loss of funding, vital projects that might have ensured better patient outcomes face setbacks or are entirely halted. These cuts not only impact the operational capabilities of Institutional Review Boards (IRBs) responsible for safeguarding participants but can also lead to a diminished pool of qualified personnel to oversee research practices. The fallout from inadequate oversight can translate into increased risks for participants, ultimately hindering the progress of essential medical advancements.
Additionally, federal research grants are foundational to establishing sound ethical practices in medical research. Cuts to these grants mean that research institutions may struggle to maintain robust IRB oversight, which is necessary for protecting the rights and welfare of study participants. The erosion of funding affects the training of IRB staff and the resources available to engage in ongoing ethical reviews, which can result in the risk of bias in studies or the overlooking of potential harms to patients. Ensuring patient safety in research is critical; without sustained funding, we jeopardize not only individual participants but also public trust in the entire research enterprise.
The Importance of Medical Research Funding
Medical research funding is crucial for advancing healthcare and understanding complex diseases. The suspension of federal research grants, such as those affected by the recent freeze at Harvard, illustrates the direct link between funding availability and research progress. Without adequate resources, research narratives stall, delaying breakthroughs that could ultimately benefit patient care. Furthermore, funding fosters collaboration between institutions, promoting shared knowledge that is essential in tackling widespread health issues. The lack of financial support can hinder innovative projects that rely on multi-site studies managed by IRBs, which ensure that ethical standards are upheld across the board.
Furthermore, federal research grants help to build confidence in the medical research process by financing studies that adhere to strict regulations and ethical guidelines. When funds are withdrawn, as seen in the case of the SMART IRB initiatives, the continuity of oversight becomes compromised, leading to potential ethical violations and human rights concerns in research practices. The financial backbone that grants provide is not merely an operational necessity; it represents a commitment to ethical standards and patient safety that must be maintained to protect those who participate in clinical research.
Challenges Posed by IRB Oversight and Funding Limitations in Research Practices and Patient Safety
One of the critical roles of (IRBs) is to ensure that research protocols meet ethical standards designed to protect participants. However, when faced with funding limitations, the effectiveness and efficiency of IRB oversight can suffer. Protocol reviews may slow down, leading to delays in launching studies that could benefit patients. The cascade of consequences from these delays can lead to an erosion of patient safety, as prolonged approval processes might mean that urgent research into new treatments is halted or deferred indefinitely.
Moreover, the financial strain experienced by IRBs due to funding cuts limits their ability to invest in training programs for their members. These training programs are vital for developing a thorough understanding of ethical guidelines and regulatory requirements governing human research. When IRB personnel lack this knowledge, their ability to scrutinize research proposals rigorously diminishes, increasing the risk for participants in those studies. This highlights the necessity of stable funding for maintaining the integrity of patient protection mechanisms in medical research.
Historical Evidence of the Need for Robust IRB Oversight in Medical Research
The past is rife with examples of medical research that lacked appropriate oversight, underscoring the importance of well-funded, functionally robust IRBs today. Events, such as the infamous Tuskegee Study, have shaped contemporary research regulations and emphasize the necessity for a vigilant oversight community. Effective IRB structures developed in response to these historical injustices; they ensure ethical compliance in studies involving human subjects, not only enhancing patient safety but also maintaining public trust in research initiatives.
Moreover, significant medical advancements often stem from collaborative studies that can benefit greatly from an effective IRB system. With all of the hurdles that can arise in multi-institutional studies, funding allows these IRBs to streamline processes and reduce bureaucratic delays that could hinder research. Robustly funded IRB initiatives are paramount in making scientific research ethical and effective, as they ensure that the lessons from past failures are not forgotten and are actively incorporated into current practices.
How Collaborative Research Efforts Suffer from Funding Cuts and IRB Limitations
Collaboration among research institutions is essential for efficiently tackling complex health issues, and yet funding cuts have severely hampered this collaborative spirit. The stoppage of major funding contracts not only affects new research but also prevents established studies from expanding and bringing new sites onboard. This lack of integration can stifle innovation, slowing momentum on critical projects intended to discover treatments for diseases such as Alzheimer’s or cancer. The landscape for collaborative research becomes very challenging when adequate funding is not in place to support both the research and its oversight.
The impact of such funding cuts reaches beyond immediate research collaborations; they can lead to long-term challenges in interdisciplinary studies that rely on multi-site IRB oversight. When institutions face delays in gaining IRB approvals due to funding restrictions, the entire research timetable is thwarted. Optimizing the capabilities of IRBs—facilitated by sustained funding—can help ensure that the vast resources of various institutions work together harmoniously for the greater good of patient safety and scientific inquiry.
Understanding Public Perception and Trust in Medical Research Amidst Funding Challenges
The perception of medical research among the public can be significantly influenced by funding and oversight practices. When funding cuts occur, public trust may be eroded if people begin to believe that research is not being conducted ethically or thoroughly. A loss of confidence in the research process can deter individuals from participating in clinical trials, which limits the diversity of study participants and ultimately impacts the generalizability of research findings. Ensuring transparency in how funding is utilized and how studies are monitored by IRBs is critical to bolstering public trust.
In essence, public perception is intricately linked to how effectively research funding is managed. As researchers face challenges brought on by funding shortcomings, it might foster skepticism about the integrity and intentions behind medical research. Initiatives that promote outreach and education, detailing how IRBs work to protect participants, as well as how funding fuels ethical research practices, may help rebuild confidence. It is imperative that stakeholders showcase the importance of maintaining strong ethical standards to reassure the public that their safety is the priority in medical research.
Future of Medical Research Funding: Directions for Advocacy and Improvement
Looking forward, it is critical to advocate for sustained, or even increased, medical research funding to ensure both patient safety and research integrity. Policymakers, academic institutions, and public health advocates need to work collaboratively to emphasize the importance of robust funding frameworks. These frameworks should aim not just to maintain existing research practices but to innovate and expand them to meet new health challenges effectively.
A multi-faceted approach to improving funding scenarios should include campaigns that illustrate the broad benefits of biomedical research, highlighting the life-saving innovations that arise from it. Engaging the public in these conversations can help galvanize support for federal research grants, laying the groundwork for a healthier future. Harnessing the collective voice of patients, researchers, and public health officials can help ensure the prioritization of patient safety in research outcomes while continuing the fight against funding cuts that threaten the advancement of medical science.
Frequently Asked Questions
How do federal research grants impact patient safety in medical research?
Federal research grants play a crucial role in enhancing patient safety in medical research by providing necessary funding for rigorous oversight and compliance with ethical guidelines. These grants enable institutions to employ Institutional Review Boards (IRBs) that ensure comprehensive review and monitoring of studies to protect participants’ rights and welfare.
What are the consequences of funding cuts on medical research and IRB oversight?
Funding cuts can severely disrupt medical research endeavors, leading to diminished IRB oversight. This situation may result in halted studies, inadequate safety protocols, and increased risks to patient safety, which ultimately undermines public trust in research.
Why is IRB oversight vital for ensuring patient safety in research funded by federal grants?
IRB oversight is essential for protecting patients in federally funded medical research, as it ensures that all studies are ethically reviewed. IRBs evaluate the risks and benefits of research proposals, ensuring that patient safety is prioritized throughout the research process.
How do funding cuts affect medical research conducted at institutions like Harvard?
Funding cuts significantly impact medical research at institutions such as Harvard by limiting access to federal research grants. This can hinder collaboration and delay essential studies aimed at improving patient safety and advancing medical knowledge.
What measures are taken to protect patient safety when federal research grants are involved?
When federal research grants are involved, measures to protect patient safety include thorough IRB reviews, informed consent processes, and continuous monitoring of ongoing studies. These procedures help mitigate potential risks associated with clinical research.
How can the impact of funding cuts on medical research funding be addressed?
Addressing the impact of funding cuts on medical research requires advocating for increased federal research grants, fostering collaboration among institutions, and ensuring that IRBs maintain their critical oversight roles to safeguard patient safety.
What is the role of IRBs in the context of federal research grants?
IRBs play a pivotal role in the context of federal research grants by ensuring that all proposed studies adhere to ethical standards and regulations, ultimately protecting the safety and welfare of research participants.
How do historical events shape the current landscape of medical research funding and patient safety?
Historical events, such as unethical medical trials, have led to the establishment of stringent laws and regulations governing medical research funding, prompting a greater emphasis on patient safety and robust IRB oversight.
What impact do delays in research studies have on patient safety in medical research?
Delays in research studies due to funding cuts can compromise patient safety by slowing down the advancement of critical medical innovations, potentially leaving patients without access to new therapies and treatments.
What are the challenges faced by medical researchers due to cuts in federal research grants?
Medical researchers face numerous challenges due to cuts in federal research grants, including reduced funding for key oversight components, limited resources for IRB activities, and potential disruptions in ongoing studies, all of which can negatively affect patient safety.
| Key Point | Details |
|---|---|
| Funding Freeze | The Trump administration froze over $2 billion in federal research grants to Harvard, disrupting oversight of medical studies. |
| SMART IRB System | The SMART IRB system facilitates oversight of multisite medical research. Its funding is crucial for patient safety and ethical compliance. |
| IRB Oversight | Institutional Review Boards (IRBs) review, approve, and oversee research involving human participants to ensure their rights and safety. |
| Impact of Funding Cuts | Cuts to funding impede patient safety efforts and can halt important research studies midstream, increasing risks to participants. |
| Historical Context | Past unethical medical experiments highlight the need for rigorous oversight provided by IRBs to protect participants. |
| Overall Consequences | Funding cuts can reinforce public skepticism toward medical research and decrease trust in research institutions. |
Summary
Medical research funding is critical for ensuring the health and safety of patients involved in clinical studies. The recent halt in funding, particularly the freeze on over $2 billion in federal grants to Harvard, disrupts the vital oversight mechanisms such as the SMART IRB system, which plays a key role in protecting participants’ rights. Such funding cuts not only jeopardize current research efforts and can bring studies to a standstill but also risk reinforcing skepticism and mistrust among the public toward the entire medical research field. Therefore, it is imperative for stakeholders to recognize the importance of continuous support for medical research funding to uphold ethical standards and participant safety.